Know us
About PHAIR
Every year 600.000 persons are admitted to Danish hospitals, of which 5% (30.000 admissions) are due to drugs’ side effects. To reduce patient harm and comply with the third WHO Global Patient Safety Challenge: Medication Without Harm this number needs to be reduced.
The aim of PHAIR is to set the basis for a national drug safety surveillance system that will increase the quality of life of patients in Denmark. We suggest a novel approach to pharmacovigilance, which could place Denmark as Europe's spearhead in real-time detection, analysing and prevention of side effects in pharmacotherapy.
Our aim is to build, collect and communicate a secure, privacy preserving national wide network which will combine primary and secondary health care data with epidemiological and AI methods, and automation. This state-of-the art system will enable real time and unbiased side effect surveillance that is far less vulnerable to labour and time intensive handling.
With Covid-19 vaccine as the test case, a treatment with unprecedented rapid roll out and socio-economic impact, the aim is to apply the above system and complement ongoing vaccine safety surveillance activities by facilitating real time analysis of side effects and potential discovery of hitherto unknown side effects.
The timeline for the project is three years. It is the project’s ambition that it will give rise to new ideas and initiatives, as PHAIR an innovative never-seen-before project and thus represents endless possibilities for researchers, IT companies and authorities.
Organisation
To accomplish the aim of PHAIR the project has secured the collaboration of a multi-disciplinary team comprising authorities, clinical epidemiologists, stakeholder representatives, IT companies, AI researchers and patient representatives. Deliverables and milestones are achieved through the established work packages covering all aspects of the project:
- (WP1) Project management
- (WP2) Legal and ethics
- (WP3) Technical infrastructure
- (WP4) Data management and analysis platform
- (WP5) Real time analyses
- (WP6) Stakeholder management and Implementation
- (WP7) Communication.
The project is governed by a Steering Committee and Implementation Board, and managed by a Project Coordination Team (PCT). Learn more about PHAIR partners below.
Behind PHAIR are the following partners:
- Bispebjerg and Frederiksberg Hospital, Department of Clinical Pharmacology
- Danish Patients
- The Danish Medicines Agency's Data Analysis Center (DAC)
- The University of Copenhagen, Department of Computer Science
- State Serum Institute, Department of Epidemiology Research
- The University of Southern Denmark, Department of Clinical Pharmacology, Pharmacy and Environmental Medicine
- Trifork A/S
PHAIR strive at combining the use of big data with artificial intelligence in an unprecedented strong research environment supervised by the country’s leading data-experts, epidemiologists and clinicians in cooperation with a leading IT-company, patient representatives and the authorities, securing a robust pathway from idea to product and clinical implementation.
For this to succeed a governance structure is rolled out which supports a) effective outreach approach and dialogue mechanisms with stakeholders and beneficiaries, b) dynamic decision-making enabling any required and timely adjustment, and c) effective management coordination structure.
Core in the governance structure is the Project Management Team, which consists of the original project creator/authors and other Work Packages leaders or main contributors. See the Project Management Team here.
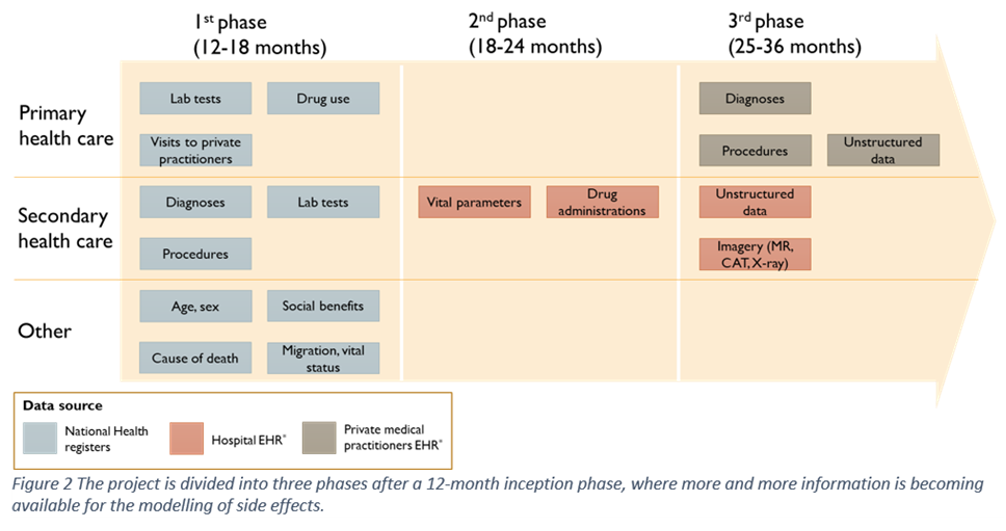
The project is divided into an inception phase and 3 analytical phases. Firstly, a 12 month inception phase where mandatory applications and approvals are applied for, synthetic data is established, machine learning and epidemiological models are tested, infrastructure is mapped and built and recruitment is consolidated. This is followed by three analytical phases. The first phase (m12-18) includes work with national register data. The second analytical phase (m18-24) will include structured information from the EHR of hospitals. The final phase (m25-36) will add EHR information from the primary health care and unstructured data (e.g. free text and images from the secondary sector) to the RWD analytical platform.
Approach
The ambition for user involvement in PHAIR is to do research with patients and citizens rather than about or for patients and citizens. The citizen’s perspective is important for PHAIR and is also fundamental for their trust in the PHAIR project and the utilisation of AI-technology.
PHAIR is a research and development project utilising AI to ultimately monitor and predict potential risks of adverse drug reactions with far greater precision and speed than current methods allow. This advancement aims to enhance the safety of medicines. Given the novelty of AI-technology, its full scope remains unclear to citizens, patients, authorities and researchers. Hence, there is a joint effort to incorporate diverse perspectives in the project's development.
To achieve this, a user panel is established under the auspices of Danish Patients, actively engaged in shaping the course of PHAIR. The purpose of the panel is to empower citizens and patients to influence the development of selected aspects of the research project. The user panel is tasked with assisting researchers with insights and in identifying the opportunities and concerns that citizens and patients may have regarding the use of large health data sets in monitoring adverse drug reactions.
The panel aims to represent the population comprehensively, considering factors such as age, gender, residence, and education. Additionally, it seeks diversity in knowledge and attitudes toward the use of AI for monitoring personal data, as well as any experiences with adverse drug reactions.
The panel and researchers meet and collaborate in several workshops. In this collaboration, dialogue and a reflective approach between citizens and patients, researchers, and authorities are crucial, as this is where mutual learning and exchange of experiences take place.
Workshops:
- Spring 2023: Recommendations for project design regarding the use of health data for monitoring side effects.
- Winter 2024: Recommendations for prioritising hypothesis testing.
- Autumn 2024 Recommendations for communicating PHAIR results to the public.
Use of health data
Overall, the user panel expressed that their trust in the project's data usage is contingent upon citizens and patients feeling secure that data will not be misused now nor in the future.
The citizens expect, that the project is transparent about the data used and data protection in general. When this is adhered to, citizens trust that the researchers know what data they need to get the most beneficial outcomes from PHAIR. Therefore, the user panel largely agree that the data intended for use in PHAIR should be included, as omitting any data may render the results uncertain.
Data protection
Before any data is used for research, we ensure compliance with the national Data Protection Act and GDPR. This ensures that personal information and rights are protected and that data is only used for its intended research purpose.
The project is managed under a joint data controllership according to GDPR between Capital Region of Denmark (Region Hovedstaden), Statens Serum Institut, University of Southern Denmark (Syddansk Universitet), University of Copenhagen (Københavns Universitet), and the Danish Medicines Agency (Lægemiddelstyrelsen). The parties have mutually agreed on the purpose, scope, and tools for handling personal data, ensuring secure and responsible data processing.
The parties have entered into a joint data responsibility agreement under Article 26(2) of the GDPR. This agreement defines the roles of each party ensuring transparency and compliance with data protection regulations. Individuals can exercise their rights with any of the responsible parties under the joint data controllership.
Additionally, a Data Protection Impact Assessment (DPIA) has been conducted by Poul Schmidt Law Firm, legal advisor to the Danish Government. The assessment ensures that risks related to personal data processing are identified and minimized. The final conclusion states that the remaining risks are low to moderate and do not pose a high risk to individual rights and freedoms.
We are committed to ensuring that all data processing is done securely, ethically, and in compliance with legal requirements.
Data access
The analysis platform developed during the project is hosted by the Danish Medicines Agency at their partition of the High-Performance Computer at The Danish National Genome Centre (NGC). NGC will manage the digital infrastructure and provide the basic infrastructure for the project in the form of a secure private cloud consisting of computing, storage, and networking. The specific task of NGC is to securely store and process data on a secure platform and allow secure access to data via an encrypted connection. Access researchers is managed by a dedicated platform administrator in accordance with documented guidelines. Only approved PHAIR researchers will have access to health data.
Logging will also occur to document who processes the personal data.
High-Performance Computer (supercomputer)
The Danish National Genome Center is a world-leading facility when it comes to handling huge amounts of diverse health data in the field of Personalised Medicine. The supercomputer lives up to the international standards for data protection, and patient data are kept and used with the most careful attention to safety (The supercomputer (ngc.dk)).
Data pseudonymisation
All data will be pseudonymised or fully anonymised where possible, and kept in a secure server certified for handling personal data. Only data necessary for the project will be gathered and will be deleted after project completion according to the relevant data approvals and legal basis.
The user panel is determined to instill trust that the data collected in PHAIR will not be used in the future for other projects with different political or commercial purposes. The panel recommends that researchers adhere to clear ethical guidelines, as this will contribute to building credibility and trust in the project as a whole. Additionally, the panel is attentive to ensuring the project's independence from vested interests and ethically considering whom to seek funding from and whom not to.
In addition to the user panel, philosophers and ethicists from the University of Copenhagen are involved in identifying complex ethical issues in the project, further enhancing transparency and focusing on relevant points of attention.
When personal data is collected solely for research and statistical purposes under the special provisions of the Data Protection Act, the information may not subsequently be used for purposes other than research and statistics, unless the results are anonymised to such an extent that they cannot be traced back to individual individuals. Ethical framework for PHAIR will be continuously developed outlining the allowed use of information. As a mitigating measure, emphasis is placed on outlining the intended uses of the information to prevent mission creep.
The ethical framework has an overall purpose to prevent data misuse for political or commercial purposes, as well as fostering credibility and trust externally.
Further, the PHAIR project is grounded upon pillars that pave the way for good ethical conduct such as transparent communication, clinician involvement, peer review and strict practices for data managing, protection and security. These pillars underscore PHAIR’s commitment to positive impact on patient care, ensuring success and benefiting the stakeholders involved.
Project coordination team

Espen Jimenez Solem
Doctor at Bispebjerg and Frederiksberg Hospital, Department of Clinical Pharmacology

Mads Nielsen
Professor in Computer Science at The University of Copenhagen, Department of Computer Science

Claus Møldrup
PhD Pharm. ex-professor at Danish Medicines Agency, Data Analytics Center
